Teche-Vermilion Long-term
Water Quality Monitoring Program
|
|
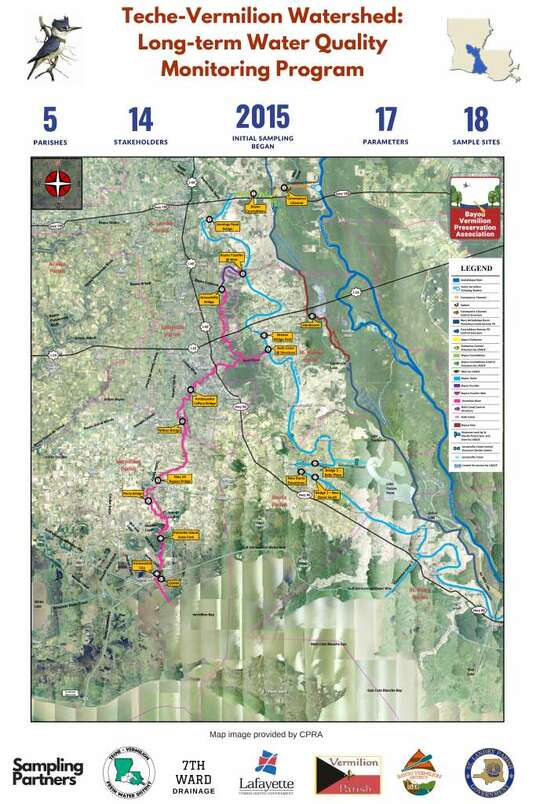
This program is administered by an inter-governmental sampling team, including the Teche-Vermilion Fresh Water District (TVFWD), LCG and BVD, who have elected to continue sampling indefinitely.
After conferring with experts at the University of Louisiana at Lafayette and the LDEQ regional office, the sampling frequency was set to once a month. The number of sites sampled has increased to eighteen. The sites extend from the headwaters of the Teche-Vermilion Watershed and down each channel, with the Teche sampling ending downstream of New Iberia and the Vermilion sampling ending at the Gulf Intracoastal Waterway. All 18 sites are tested for fecal coliform counts on a monthly basis. Site conditions are noted at each sampling location:
Parameters collected in the field at each sampling location, using a YSI meter:
Four out of the ten sites conduct lab analyses for the following parameters:
2018 Special Sampling Event: DNA sampling of fecal coliform indicator bacteria Two locations were in Lafayette Parish, one location in St. Landry and another in Vermilion Parish. Four sources of fecal coliform indicators were tested: Humans, dogs, birds and cows. Results showed that fecal coliform indicators found in August of 2017 were from human and dog sources. DNA of cows and birds were not discovered during this sampling event. |
Water Quality Parameters
Fecal coliform bacteria
What is it?
Fecal coliform bacteria are found in the feces of human beings and other warm-blooded
animals. By themselves, fecal coliform bacteria do not usually cause disease. In fact, they are
already inside you. They occur naturally in the human digestive tract and aid in the digestion
of food.
However, when a human being or other warm-blooded animal is infected with disease,
pathogenic (disease causing) organisms are found along with fecal coliform bacteria.
Why does fecal coliform matter?
Think of high levels of fecal coliform bacteria as a warning sign that water can make you sick,
rather than as a cause of illness. If fecal coliform counts are high (over 400 colonies/100 ml of a
water sample) in a body of water, there is a greater chance that disease-causing organisms are
also present. If you are swimming in waters with high levels of fecal coliform, you have a
greater chance of developing a fever, nausea or stomach cramps from swallowing disease causing
organisms, or from pathogens entering the body through cuts in the skin, the nose, mouth, or ears. Some examples of diseases and illnesses that can be contracted in water with high fecal coliform counts include typhoid fever, hepatitis, gastroenteritis, dysentery, and ear infections.
Fecal coliform bacteria are living organisms, unlike the other conventional water quality
parameters. The fecal coliform bacteria multiply rapidly when conditions are good for growth
and die in large quantities when they are not.
How does fecal coliform get in the water?
Untreated sewage, poorly maintained septic systems, un-scooped pet waste, and farm animals
with direct access to streams can cause high levels of fecal coliform bacteria to appear in a
water body.
Temperature
What is it?
Temperature is a measure of how much heat is present in the water.
Why does temperature matter?
Water temperature tells many things about the health of a river. Temperature affects:
1. Dissolved oxygen levels in water –Cold water holds more oxygen than warm water.
2. Photosynthesis –As temperature goes up, the rate of photosynthesis and plant growth
also goes up. More plants grow and more plants die. When plants die, decomposers
eat them and use oxygen. So when the rate of photosynthesis increases, the amount
of oxygen needed by aquatic organisms increases.
3. Animal survival –Many animals need certain temperatures to live. For example,
stonefly nymphs and trout need cool temperatures. Dragonfly nymphs and carp can live
in warmer water. If water temperatures change too much, many organisms can no
longer survive.
4. Sensitivity to toxic wastes and disease –Wastes often raise water temperatures.
This leads to lower oxygen levels and weakens many fish and insects. Weakened
animals get sick and die more easily.
How does water get warmer?
In the summer, the sun heats up sidewalks, parking lots and streets. Rain falls on these areas,
warms up, and runs into the river. Factories and stations that generate electricity to cool their
processes also use water. Warm water enters the river, raises the temperature of the
downstream area and changes oxygen levels. These are forms of thermal pollution. Thermal
pollution is one of the most serious ways humans affect rivers.
Cutting down trees along the bank of a river or pond also raises water temperature. Trees help
shade the river from the sun. When they are cut down, the sun shines directly on the water and
warms it up. Cutting down trees also leads to erosion. When soil from the riverbank washes
into the river the water becomes muddy (turbid). The darker, turbid water captures more heat
from the sun than clear water does. Even murky green water with lots of algae will be warmer
than clear water.
Dissolved Oxygen (DO)
What is it?
Like people, aquatic organisms need oxygen to survive and stay healthy. In areas with waves, or
where water tumbles over rocks, falling water traps oxygen and mixes it into the water.
Unlike people, aquatic organisms breathe oxygen that is dissolved in water. To breathe
underwater, fish and other aquatic organisms use gills instead of lungs. These gills breathe the
oxygen dissolved in the water. As you know, a fish out of the water will die because it can no
longer breathe.
Why does DO matter?
Imagine living in a place with polluted air. As the air quality becomes worse, the health of the
people who live there becomes worse. The same is true in water. Clean, healthy water has
plenty of DO. When water quality decreases, DO levels drop and it becomes impossible for
many animals to survive. Some fish such as trout require lots of dissolved oxygen. Others such
as carp can live in water with lower levels of DO.
Warmer water holds less oxygen than cold water. Also, the time of year and many other factors
affect the amount of DO in water.
How do DO levels in the water drop?
The main reason DO levels might fall is the presence of organic waste. Organic waste comes
from something living or that was once living. It comes from raw or poorly treated sewage;
runoff from farms and animal feedlots; and natural sources like decaying aquatic plants and
animals and fallen leaves in water.
Microscopic organisms, called decomposers, break down the organic waste and use oxygen in
the process. Two common types of decomposers are bacteria and protozoa. More waste means
more decomposers and more oxygen being used.
DO levels can also fall due to any human activity that heats the water.
Biochemical Oxygen Demand (BOD)
What is it?
When organic matter decomposes, microorganisms (such as bacteria and fungi) feed upon this
decaying material and eventually the matter becomes oxidized. Biochemical oxygen demand,
or BOD, measures the amount of oxygen consumed by microorganisms in the process of
decomposing organic matter in stream water. The harder the microorganisms work, the more
oxygen they use, and the higher the measure of BOD, leaving less oxygen for other life in
the water.
Why does high BOD matter?
BOD directly affects the amount of dissolved oxygen in rivers and streams. The more rapidly
oxygen is depleted in the stream, the greater the BOD. This means less oxygen is available for
other aquatic life, such as insects and fish. A high BOD measure harms stream health in the
same ways as low dissolved oxygen: aquatic organisms become stressed, suffocate, and die.
The few organisms that can survive with less oxygen, like carp and sewage worms, will increase
in number.
How do BOD levels rise?
As more organic matter enters a stream, the BOD will rise. Organic matter may include leaves
and woody debris; dead plants and animals; animal manure; effluents from pulp and paper mills,
wastewater treatment plants, feedlots, and food-processing plants; failing septic systems; and
urban storm water runoff.
Total Phosphorous
What is it?
Phosphorus is a nutrient found in all living things. It is also a mineral in nature. Both plants and
animals have phosphorus in their bodies. It is in most of the foods we eat. When people buy
fertilizer for their gardens, they use nutrients such as phosphorus to help plants grow.
Why does total phosphorous matter?
Scientists believe that when too much phosphorus enters a river or lake, plants grow more.
Tiny plants like algae use the phosphorus to grow. Other plants that live on the surface and
bottom of a river or lake use phosphorus also. When plant growth increases, the water turns
pea-green and becomes cloudy. The green color comes from the chlorophyll content of the
tiny floating plants.
Too many plants living in the water can lead to some bad results. When these plants die (which,
in the case of tiny plants or algae, is very often), they sink to the bottom. There, bacteria
decompose the dead plant parts. They use up most of the oxygen in the water. They actually
use more oxygen than the amount added by the plants through photosynthesis. Therefore, too
many plants in the water from too much phosphorus leads to less oxygen. This is what happens
when too much phosphorus enters the water:
1. Phosphorus enters the water
2. Plants take up the phosphorus and grow too much
3. Plants (algae) die and sink to the bottom
4. Bacteria at the bottom decompose the dead plants, using up oxygen in the process
5. Oxygen levels drop, killing fish or aquatic insects
6. Phosphorus continues to enter the water
7. The cycle continues
How does too much phosphorous get in the water?
Phosphorus enters the water from a number of places. It is found when human and animal
wastes are flushed into waterways, either from poorly treated sewage, broken pipes or runoff.
Some industrial wastes also carry phosphorus into the water. Whenever trees and grass are
removed from an area, soil erodes into waterways, carrying the phosphorus that sticks to soil.
Fertilizers used at homes on lawns and on farm fields carry much of the phosphorus in the
fertilizer into streams when it rains. Since rivers flow, the phosphorus is carried downstream.
Lakes do not flow like rivers but trap nutrients instead. Therefore, high levels of phosphorus
are more serious in lakes and ponds.
Nitrogen
What is it?
Nitrogen is one of the most common elements in the world. All living plants and animals need it
to build proteins. Nitrogen and phosphorus are both nutrients.
Why does nitrogen matter?
High levels of nitrogen may make some people sick, especially young babies. This happens to
people who drink directly from groundwater wells where the water has too much nitrogen.
Because nitrogen is a nutrient like phosphorus, the effects of this nutrient on water are almost
the same. Like phosphorus, extra nitrogen in water leads to rapid plant growth. Too many
plants living in the water can lead to some bad results. When these plants die (which, in the
case of tiny plants or algae, is very often), they sink to the bottom. There, bacteria decompose
the dead plant parts. They use up most of the oxygen in the water. They actually use more
oxygen than the amount added by the plants through photosynthesis. Therefore, too many
plants in the water from too much phosphorus leads to less oxygen. This is what happens when
too much nitrogen enters the water:
1. Nitrogen enters the water
2. Plants take up the nitrogen and grow and grow and grow
3. Plants (algae) die and sink to the bottom
4. Bacteria at the bottom decompose the dead plants, using up oxygen in the process
5. Oxygen levels drop, killing fish or aquatic insects
6. Nitrogen continues to enter the water
7. The cycle continues
How does too much nitrogen get in the water?
Nitrogen can be found in fertilizers and in human or farm animal wastes. In some cases, home
septic systems in rural areas leak waste into the ground. This waste should be filtered by the
soil around the septic system. However, this does not always happen. Therefore, groundwater
can become polluted by nitrogen in the wastewater.
pH
What is it?
pH is a measurement of the acidity or basic quality of water. For example, lemons, oranges and
vinegar are high in acid (“very acidic”). Acids can sting or burn, which is what you feel when
you eat some kinds of fruit with a sore in your mouth. The pH scale ranges from a value of 0
(very acidic) to 14 (very basic), with 7 being neutral. The pH of natural water is usually between
6.5 and 8.2
Why does the pH level matter?
At extremely high or low pH levels (above 9.6 or below 4.5), the water becomes unsuitable for
most organisms. Young fish and insects are also very sensitive to changes in pH. Most aquatic
organisms adapt to a specific pH level and may die if the pH of the water changes even slightly.
How do levels of pH become too high or low?
pH can vary from its normal levels (6.5 to 8.2) due to pollution from automobiles and coal burning
power plants. These sources of pollution help form acid rain. Acid forms when
chemicals in the air combine with moisture in the atmosphere. It falls to earth as acid rain or
snow. Many lakes in eastern Canada, the northeastern US, and northern Europe are
becoming acidic because they are downwind of polluting industrial plants. Drainage from
mines can seep into streams and ground water and make the water more acidic as well.
Turbidity
What is it?
Think of turbidity as the opposite of clarity. It is a measure of how cloudy a water body is. Most
people have seen how rivers turn brown after a heavy rain. Soil particles carried by runoff cause
this to happen.
Why do high levels of turbidity matter?
High amounts of soil in the water will block sunlight from reaching the bottom of a river or a
lake in shallow water. When the water is turbid, floating particles absorb heat from the sun
and cause the water temperature to rise. Higher temperatures cause oxygen levels in the
water to fall, limiting the ability of fish and insects to survive there.
Another effect is that the floating particles may clog fish gills. When these particles sink, they
can smother and kill fish and aquatic insect eggs that lay on the bottom. Turbidity can also limit
plant growth. This happens when sunlight cannot reach the plants’ leaves.
The combination of warmer water, less light and oxygen depletion makes it impossible for some
forms of aquatic life to survive.
How do turbidity levels rise?
Higher turbidity can be caused by human activity like cutting trees and removing vegetation
next to a body of water. Trees provide shade to keep the water cooler, and trees and other
plants help block mud and soil from washing into the water. When roads and parking lots are
constructed without the proper silt fencing, more soil and mud are likely to reach the water.
Fecal coliform bacteria
What is it?
Fecal coliform bacteria are found in the feces of human beings and other warm-blooded
animals. By themselves, fecal coliform bacteria do not usually cause disease. In fact, they are
already inside you. They occur naturally in the human digestive tract and aid in the digestion
of food.
However, when a human being or other warm-blooded animal is infected with disease,
pathogenic (disease causing) organisms are found along with fecal coliform bacteria.
Why does fecal coliform matter?
Think of high levels of fecal coliform bacteria as a warning sign that water can make you sick,
rather than as a cause of illness. If fecal coliform counts are high (over 400 colonies/100 ml of a
water sample) in a body of water, there is a greater chance that disease-causing organisms are
also present. If you are swimming in waters with high levels of fecal coliform, you have a
greater chance of developing a fever, nausea or stomach cramps from swallowing disease causing
organisms, or from pathogens entering the body through cuts in the skin, the nose, mouth, or ears. Some examples of diseases and illnesses that can be contracted in water with high fecal coliform counts include typhoid fever, hepatitis, gastroenteritis, dysentery, and ear infections.
Fecal coliform bacteria are living organisms, unlike the other conventional water quality
parameters. The fecal coliform bacteria multiply rapidly when conditions are good for growth
and die in large quantities when they are not.
How does fecal coliform get in the water?
Untreated sewage, poorly maintained septic systems, un-scooped pet waste, and farm animals
with direct access to streams can cause high levels of fecal coliform bacteria to appear in a
water body.
Temperature
What is it?
Temperature is a measure of how much heat is present in the water.
Why does temperature matter?
Water temperature tells many things about the health of a river. Temperature affects:
1. Dissolved oxygen levels in water –Cold water holds more oxygen than warm water.
2. Photosynthesis –As temperature goes up, the rate of photosynthesis and plant growth
also goes up. More plants grow and more plants die. When plants die, decomposers
eat them and use oxygen. So when the rate of photosynthesis increases, the amount
of oxygen needed by aquatic organisms increases.
3. Animal survival –Many animals need certain temperatures to live. For example,
stonefly nymphs and trout need cool temperatures. Dragonfly nymphs and carp can live
in warmer water. If water temperatures change too much, many organisms can no
longer survive.
4. Sensitivity to toxic wastes and disease –Wastes often raise water temperatures.
This leads to lower oxygen levels and weakens many fish and insects. Weakened
animals get sick and die more easily.
How does water get warmer?
In the summer, the sun heats up sidewalks, parking lots and streets. Rain falls on these areas,
warms up, and runs into the river. Factories and stations that generate electricity to cool their
processes also use water. Warm water enters the river, raises the temperature of the
downstream area and changes oxygen levels. These are forms of thermal pollution. Thermal
pollution is one of the most serious ways humans affect rivers.
Cutting down trees along the bank of a river or pond also raises water temperature. Trees help
shade the river from the sun. When they are cut down, the sun shines directly on the water and
warms it up. Cutting down trees also leads to erosion. When soil from the riverbank washes
into the river the water becomes muddy (turbid). The darker, turbid water captures more heat
from the sun than clear water does. Even murky green water with lots of algae will be warmer
than clear water.
Dissolved Oxygen (DO)
What is it?
Like people, aquatic organisms need oxygen to survive and stay healthy. In areas with waves, or
where water tumbles over rocks, falling water traps oxygen and mixes it into the water.
Unlike people, aquatic organisms breathe oxygen that is dissolved in water. To breathe
underwater, fish and other aquatic organisms use gills instead of lungs. These gills breathe the
oxygen dissolved in the water. As you know, a fish out of the water will die because it can no
longer breathe.
Why does DO matter?
Imagine living in a place with polluted air. As the air quality becomes worse, the health of the
people who live there becomes worse. The same is true in water. Clean, healthy water has
plenty of DO. When water quality decreases, DO levels drop and it becomes impossible for
many animals to survive. Some fish such as trout require lots of dissolved oxygen. Others such
as carp can live in water with lower levels of DO.
Warmer water holds less oxygen than cold water. Also, the time of year and many other factors
affect the amount of DO in water.
How do DO levels in the water drop?
The main reason DO levels might fall is the presence of organic waste. Organic waste comes
from something living or that was once living. It comes from raw or poorly treated sewage;
runoff from farms and animal feedlots; and natural sources like decaying aquatic plants and
animals and fallen leaves in water.
Microscopic organisms, called decomposers, break down the organic waste and use oxygen in
the process. Two common types of decomposers are bacteria and protozoa. More waste means
more decomposers and more oxygen being used.
DO levels can also fall due to any human activity that heats the water.
Biochemical Oxygen Demand (BOD)
What is it?
When organic matter decomposes, microorganisms (such as bacteria and fungi) feed upon this
decaying material and eventually the matter becomes oxidized. Biochemical oxygen demand,
or BOD, measures the amount of oxygen consumed by microorganisms in the process of
decomposing organic matter in stream water. The harder the microorganisms work, the more
oxygen they use, and the higher the measure of BOD, leaving less oxygen for other life in
the water.
Why does high BOD matter?
BOD directly affects the amount of dissolved oxygen in rivers and streams. The more rapidly
oxygen is depleted in the stream, the greater the BOD. This means less oxygen is available for
other aquatic life, such as insects and fish. A high BOD measure harms stream health in the
same ways as low dissolved oxygen: aquatic organisms become stressed, suffocate, and die.
The few organisms that can survive with less oxygen, like carp and sewage worms, will increase
in number.
How do BOD levels rise?
As more organic matter enters a stream, the BOD will rise. Organic matter may include leaves
and woody debris; dead plants and animals; animal manure; effluents from pulp and paper mills,
wastewater treatment plants, feedlots, and food-processing plants; failing septic systems; and
urban storm water runoff.
Total Phosphorous
What is it?
Phosphorus is a nutrient found in all living things. It is also a mineral in nature. Both plants and
animals have phosphorus in their bodies. It is in most of the foods we eat. When people buy
fertilizer for their gardens, they use nutrients such as phosphorus to help plants grow.
Why does total phosphorous matter?
Scientists believe that when too much phosphorus enters a river or lake, plants grow more.
Tiny plants like algae use the phosphorus to grow. Other plants that live on the surface and
bottom of a river or lake use phosphorus also. When plant growth increases, the water turns
pea-green and becomes cloudy. The green color comes from the chlorophyll content of the
tiny floating plants.
Too many plants living in the water can lead to some bad results. When these plants die (which,
in the case of tiny plants or algae, is very often), they sink to the bottom. There, bacteria
decompose the dead plant parts. They use up most of the oxygen in the water. They actually
use more oxygen than the amount added by the plants through photosynthesis. Therefore, too
many plants in the water from too much phosphorus leads to less oxygen. This is what happens
when too much phosphorus enters the water:
1. Phosphorus enters the water
2. Plants take up the phosphorus and grow too much
3. Plants (algae) die and sink to the bottom
4. Bacteria at the bottom decompose the dead plants, using up oxygen in the process
5. Oxygen levels drop, killing fish or aquatic insects
6. Phosphorus continues to enter the water
7. The cycle continues
How does too much phosphorous get in the water?
Phosphorus enters the water from a number of places. It is found when human and animal
wastes are flushed into waterways, either from poorly treated sewage, broken pipes or runoff.
Some industrial wastes also carry phosphorus into the water. Whenever trees and grass are
removed from an area, soil erodes into waterways, carrying the phosphorus that sticks to soil.
Fertilizers used at homes on lawns and on farm fields carry much of the phosphorus in the
fertilizer into streams when it rains. Since rivers flow, the phosphorus is carried downstream.
Lakes do not flow like rivers but trap nutrients instead. Therefore, high levels of phosphorus
are more serious in lakes and ponds.
Nitrogen
What is it?
Nitrogen is one of the most common elements in the world. All living plants and animals need it
to build proteins. Nitrogen and phosphorus are both nutrients.
Why does nitrogen matter?
High levels of nitrogen may make some people sick, especially young babies. This happens to
people who drink directly from groundwater wells where the water has too much nitrogen.
Because nitrogen is a nutrient like phosphorus, the effects of this nutrient on water are almost
the same. Like phosphorus, extra nitrogen in water leads to rapid plant growth. Too many
plants living in the water can lead to some bad results. When these plants die (which, in the
case of tiny plants or algae, is very often), they sink to the bottom. There, bacteria decompose
the dead plant parts. They use up most of the oxygen in the water. They actually use more
oxygen than the amount added by the plants through photosynthesis. Therefore, too many
plants in the water from too much phosphorus leads to less oxygen. This is what happens when
too much nitrogen enters the water:
1. Nitrogen enters the water
2. Plants take up the nitrogen and grow and grow and grow
3. Plants (algae) die and sink to the bottom
4. Bacteria at the bottom decompose the dead plants, using up oxygen in the process
5. Oxygen levels drop, killing fish or aquatic insects
6. Nitrogen continues to enter the water
7. The cycle continues
How does too much nitrogen get in the water?
Nitrogen can be found in fertilizers and in human or farm animal wastes. In some cases, home
septic systems in rural areas leak waste into the ground. This waste should be filtered by the
soil around the septic system. However, this does not always happen. Therefore, groundwater
can become polluted by nitrogen in the wastewater.
pH
What is it?
pH is a measurement of the acidity or basic quality of water. For example, lemons, oranges and
vinegar are high in acid (“very acidic”). Acids can sting or burn, which is what you feel when
you eat some kinds of fruit with a sore in your mouth. The pH scale ranges from a value of 0
(very acidic) to 14 (very basic), with 7 being neutral. The pH of natural water is usually between
6.5 and 8.2
Why does the pH level matter?
At extremely high or low pH levels (above 9.6 or below 4.5), the water becomes unsuitable for
most organisms. Young fish and insects are also very sensitive to changes in pH. Most aquatic
organisms adapt to a specific pH level and may die if the pH of the water changes even slightly.
How do levels of pH become too high or low?
pH can vary from its normal levels (6.5 to 8.2) due to pollution from automobiles and coal burning
power plants. These sources of pollution help form acid rain. Acid forms when
chemicals in the air combine with moisture in the atmosphere. It falls to earth as acid rain or
snow. Many lakes in eastern Canada, the northeastern US, and northern Europe are
becoming acidic because they are downwind of polluting industrial plants. Drainage from
mines can seep into streams and ground water and make the water more acidic as well.
Turbidity
What is it?
Think of turbidity as the opposite of clarity. It is a measure of how cloudy a water body is. Most
people have seen how rivers turn brown after a heavy rain. Soil particles carried by runoff cause
this to happen.
Why do high levels of turbidity matter?
High amounts of soil in the water will block sunlight from reaching the bottom of a river or a
lake in shallow water. When the water is turbid, floating particles absorb heat from the sun
and cause the water temperature to rise. Higher temperatures cause oxygen levels in the
water to fall, limiting the ability of fish and insects to survive there.
Another effect is that the floating particles may clog fish gills. When these particles sink, they
can smother and kill fish and aquatic insect eggs that lay on the bottom. Turbidity can also limit
plant growth. This happens when sunlight cannot reach the plants’ leaves.
The combination of warmer water, less light and oxygen depletion makes it impossible for some
forms of aquatic life to survive.
How do turbidity levels rise?
Higher turbidity can be caused by human activity like cutting trees and removing vegetation
next to a body of water. Trees provide shade to keep the water cooler, and trees and other
plants help block mud and soil from washing into the water. When roads and parking lots are
constructed without the proper silt fencing, more soil and mud are likely to reach the water.